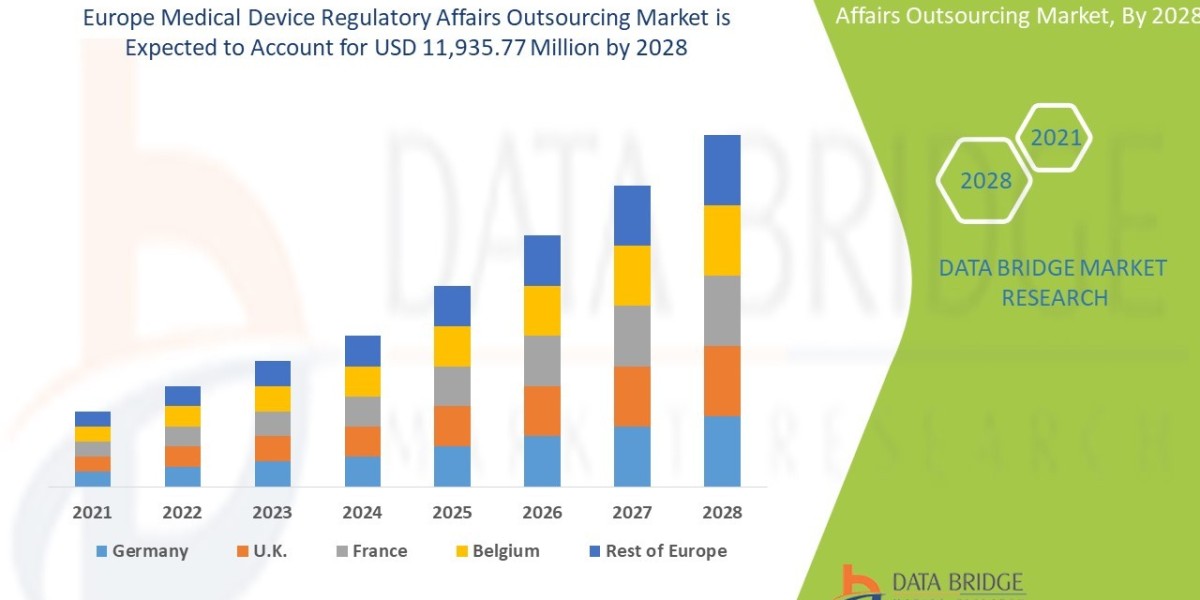
Executive Summary Europe Medical Device Regulatory Affairs Outsourcing Market :
CAGR Value
The medical device regulatory affairs outsourcing market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses that the market is growing with a CAGR of 12.8% in the forecast period of 2021 to 2028 and is expected to reach USD 11,935.77 million by 2028.
The whole Europe Medical Device Regulatory Affairs Outsourcing Market document can be divided into four major areas which include market definition, market segmentation, competitive analysis and research methodology. Important industry trends, market size, market share estimates are analysed and mentioned in the report. This Market report helps the firm in exploring new uses and new markets for its existing products and thereby, increasing the demand for its products. The market report offers an in-depth overview of product specification, technology, product type and production analysis considering major factors such as revenue, cost, and gross margin. The winning Europe Medical Device Regulatory Affairs Outsourcing Market report is comprehensive and opens a door of international market for the products.
An all-inclusive Europe Medical Device Regulatory Affairs Outsourcing Market study consists of a market attractiveness analysis, wherein each segment is benchmarked based on its market size, growth rate, and general attractiveness. The report is based on the market type, organization size, availability on-premises and the end-users’ organization type, and the availability in areas such as North America, South America, Europe, Asia-Pacific and Middle East & Africa. The info covered helps businesses know how patents, licensing agreements and other legal restrictions affect the manufacture and sale of the firm’s products. An influential Europe Medical Device Regulatory Affairs Outsourcing Market report reveals the nature of demand for the firm’s product to know if the demand for the product is constant or seasonal.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Europe Medical Device Regulatory Affairs Outsourcing Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/europe-medical-device-regulatory-affairs-outsourcing-market
Europe Medical Device Regulatory Affairs Outsourcing Market Overview
**Segments**
- **Service Type**: The Europe medical device regulatory affairs outsourcing market is segmented by service type into regulatory writing and publishing, regulatory submission, clinical trial applications, regulatory consulting, and others. Regulatory writing and publishing involve the preparation of documents required for regulatory submissions, while regulatory submission services focus on the actual submission process to regulatory authorities. Clinical trial applications services assist in the submission of applications for clinical trials, ensuring compliance with regulatory requirements. Regulatory consulting services provide expert advice and guidance on regulatory matters, helping companies navigate complex regulatory landscapes.
- **Regulatory Affairs Management**: This segment covers services related to the overall management of regulatory affairs processes, including strategy development, compliance monitoring, and risk management. Outsourcing regulatory affairs management allows companies to leverage the expertise of external partners to ensure regulatory compliance and streamline operations.
- **Product Type**: The market is further segmented by product type into in-vitro diagnostic (IVD) devices, medical imaging devices, cardiovascular devices, orthopedic devices, dental devices, ophthalmic devices, and others. Each of these product categories has unique regulatory requirements and challenges, requiring specialized expertise for successful market entry and compliance.
**Market Players**
- **ICON plc**: A global provider of outsourced drug and device development and commercialization services, ICON offers a range of regulatory affairs outsourcing services to support medical device companies in navigating global regulatory requirements.
- **IQVIA**: As a leading provider of advanced analytics, technology solutions, and contract research services to the life sciences industry, IQVIA offers regulatory affairs outsourcing services to help medical device companies navigate complex regulatory landscapes and achieve market access.
- **Parexel International Corporation**: With a focus on providing biopharmaceutical services, including regulatory affairs support, Parexel offers outsourced regulatory affairs services to medical device companies seeking to streamline regulatory processes and ensure compliance with global regulations.
- **Pharmaceutical Product Development (PPD)**: PPD offers a range of regulatory affairs outsourcing services to support medical device companies in meeting regulatory requirements and gaining market approval for their products. With expertise in regulatory strategy, submissions, and compliance, PPD helps companies navigate the regulatory landscape efficiently.
The Europe medical device regulatory affairs outsourcing market is witnessing significant growth due to the increasing complexity of regulatory requirements and the need for specialized expertise to navigate the regulatory landscape effectively. Market players such as ICON plc, IQVIA, Parexel International Corporation, and Pharmaceutical Product Development (PPD) are key contributors to the market, offering a wide range of regulatory affairs outsourcing services to support medical device companies in achieving regulatory compliance and market access. These companies play a crucial role in helping businesses streamline regulatory processes, develop effective regulatory strategies, and ensure adherence to global regulations.
One key trend shaping the Europe medical device regulatory affairs outsourcing market is the growing emphasis on compliance with evolving regulatory standards. Regulatory bodies are continuously updating and revising regulations to ensure the safety and efficacy of medical devices, creating challenges for companies to keep pace with these changes. As a result, there is a rising demand for outsourcing services that provide expertise in interpreting and implementing regulatory requirements, helping companies avoid compliance issues and expedite market entry.
Another trend impacting the market is the increasing focus on specialized regulatory services tailored to different product categories. Medical devices span a wide range of product types, each with its unique regulatory considerations and challenges. As a result, there is a growing need for regulatory affairs outsourcing providers to offer specialized services for in-vitro diagnostic devices, medical imaging devices, cardiovascular devices, orthopedic devices, dental devices, ophthalmic devices, and other product categories. By leveraging expertise in specific product areas, outsourcing partners can deliver tailored solutions that address the unique regulatory requirements of each product type.
Furthermore, the market is witnessing a shift towards strategic regulatory affairs management outsourcing. Companies are looking to outsource not just specific regulatory tasks but the overall management of regulatory affairs processes, including strategy development, compliance monitoring, and risk management. By partnering with experienced regulatory affairs management providers, medical device companies can benefit from strategic guidance, proactive compliance monitoring, and efficient risk mitigation strategies, ultimately leading to smoother regulatory approvals and market access.
Overall, the Europe medical device regulatory affairs outsourcing market is poised for continued growth and innovation as companies seek to navigate the evolving regulatory landscape efficiently. Market players will need to stay ahead of regulatory changes, offer specialized services for different product categories, and provide strategic regulatory affairs management solutions to meet the evolving needs of medical device companies in the region. By addressing these trends and challenges, regulatory affairs outsourcing providers can play a vital role in supporting the growth and success of the medical device industry in Europe.The Europe medical device regulatory affairs outsourcing market is undergoing significant growth driven by the increasing complexity of regulatory requirements and the essential need for specialized expertise to navigate the regulatory landscape effectively. Market players such as ICON plc, IQVIA, Parexel International Corporation, and Pharmaceutical Product Development (PPD) are pivotal contributors to the market, offering a plethora of regulatory affairs outsourcing services to assist medical device companies in attaining regulatory compliance and market access. These industry giants play a critical role in aiding businesses in streamlining regulatory procedures, crafting effective regulatory strategies, and ensuring conformity to global regulations.
One noteworthy trend shaping the Europe medical device regulatory affairs outsourcing market is the heightened emphasis on compliance with evolving regulatory standards. Regulatory bodies are consistently revising and updating regulations to guarantee the safety and efficacy of medical devices, posing challenges for companies to keep up with these modifications. Consequently, there is an escalating demand for outsourcing services that proffer expertise in interpreting and implementing regulatory requirements, aiding companies in averting compliance issues and expediting market entry.
Moreover, the market is witnessing a surge in the focus on specialized regulatory services tailored to different product categories. Medical devices encompass a vast array of product types, each presenting unique regulatory considerations and hurdles. Hence, there is an increasing necessity for regulatory affairs outsourcing providers to deliver specialized services for various product categories such as in-vitro diagnostic devices, medical imaging devices, cardiovascular devices, orthopedic devices, dental devices, ophthalmic devices, and others. By harnessing expertise in specific product domains, outsourcing partners can furnish tailored solutions that cater to the distinctive regulatory requirements of each product type.
Furthermore, a shift towards strategic regulatory affairs management outsourcing is being observed in the market. Companies are inclined towards outsourcing not solely specific regulatory tasks but the comprehensive management of regulatory affairs processes, encompassing strategy development, compliance monitoring, and risk management. By collaborating with seasoned regulatory affairs management providers, medical device companies can benefit from strategic guidance, proactive compliance monitoring, and efficient risk mitigation strategies, ultimately leading to smoother regulatory approvals and improved market access.
In conclusion, the Europe medical device regulatory affairs outsourcing market is poised for continuous growth and innovation as companies strive to navigate the evolving regulatory landscape effectively. Industry players must stay abreast of regulatory changes, offer specialized services for distinct product categories, and provide strategic regulatory affairs management solutions to cater to the evolving requirements of medical device companies in the region. By addressing these trends and challenges, regulatory affairs outsourcing providers can play a pivotal role in bolstering the growth and prosperity of the medical device industry in Europe.
The Europe Medical Device Regulatory Affairs Outsourcing Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/europe-medical-device-regulatory-affairs-outsourcing-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Key Questions Answered in This Report: –
- How has this Europe Medical Device Regulatory Affairs Outsourcing Marketperformed so far and how will it perform in the coming years?
- Which are the key product types available in this Europe Medical Device Regulatory Affairs Outsourcing Market?
- Which are the major application areas in theEurope Medical Device Regulatory Affairs Outsourcing Market?
- What are the key distribution channels in the global Europe Medical Device Regulatory Affairs Outsourcing Market?
- What are the key regions in this Europe Medical Device Regulatory Affairs Outsourcing Market?
- What are the price trends?
- What are the various stages in the value chain of this industry?
- What are the key driving factors and challenges in the market?
Browse More Reports:
Global Black Tea Ingredients Market
Global Bean-To-Bar Chocolate Market
Global Ceramic Microspheres Market
Global Sensor Signal Conditioner (SSC) ICs Market
North America Intraoperative Imaging Market
Global Veneer Sheets Market
Middle East and Africa Infection Control Market
Global Privileged identity management Market
Global Solid-State Car Battery Market
North America, Europe and Asia-Pacific Additive Manufacturing Market
Global Citrate and Citrate Salts Market
Global Processed Meat Market
Global Essential Oils Market
North America Molecular Diagnostics Market
Global Logistics Nodes Market
Global Azospirillum Bacteria Fertilizers Market
Global Garage Body Shop Equipment Market
Global Biotechnology Reagents Market
Global Osteomyelitis Market
Global Active Sensor Market
Global Examination Glove Market
North America Discharge Inks in Textile Industry Market
Middle East and Africa Premium Chocolate Market
Global Radio Frequency (RF) Power Semiconductor Market
Global Quick Response (QR) Code Label Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- [email protected]